Message
Welcome to all readers on the Web Site of Hirabara Engineering Service Ltd. (HES)

HES will make a determined effort to contribute to the customer’s satisfaction of needs through “users’ engineering”, which is aimed at any performance in every aspect of users’ needs.
HES has dealt with engineering services mainly for planning and designing as consultancy about new and remodeling of pharmaceutical manufacturing and research facilities based on GxP with validation and calibration, since it was founded in September, 2012.
We are convinced that maintaining “the key service functions of reviewing and advising the whole management in proper and long customer’s perspective” based on the corporate management principle of “evolving engineering service from a customer’s viewpoint” could lead to the sustainability of the organization.
The company has organized the local business framework to fulfill prompt and accurate service function to meet the requirements demanded by pharmaceutical companies and other new comers into the pharmaceutical industry in Hokuriku, since Hokuriku business office was established in Toyama prefecture in spring 2015,
We could utilize abundant experiences in the schematic design / preliminary design / compliance with relevant regulations and would execute business development of engineering service for customer satisfaction.
We would be grateful for your continued supports and cooperation.
President and Representative Director Hideyuki Sakakibara
Company
| Company name | Hirabara Engineering Service Ltd. |
|---|---|
| President and Representative Director | Hideyuki Sakakibara |
| Director | Eiju Akiyama |
| Establishment | September 5, 2012 |
| Head Office | Dai-ichi Seimei Bldg.4F, 4-59, Bentendori, Naka-ku, Yokohama 231-0007, Japan Tel:+81 45 323-9323 Fax:+81 45 323-9324 |
| Hokuriku Branch | La Tour 803, 1-7-17, Sakuramachi, Toyama 930-0003,Japan Tel:+81 76 482-4750 Fax:+81 76 482-4749 |
Business contents
Consultation and engineering services relevant to the construction and operation of various kinds of pharmaceutical manufacturing and research facilities
Corporate Management Philosophy
Evolving “the application of users’ engineering” to the construction of pharmaceutical / research facility.
Hirabara Engineering Service Ltd. (HES) proffers the consultant and engineering service to support the accomplishment of any construction and renovation project in the pharmaceutical industry.
Our corporate motto is to fulfill the function on technology-oriented “user’s engineering”.
In other words, HES business on “user’s engineering” is customer-oriented engineering service.
We are sure to lead the sound and successful completion of pharmaceutical-related facility construction wherein customers want ideal configuration throughout the project under actual circumstances.
Service
Consultation Service
Realistic consultation matching the actual circumstances every customer
HES helps to offer any practical or feasible solution of various challenging difficulties as a member of customers, which encounter when the pharmaceutical or research facilities are realistically constructed or operated.
Service outlined
- Detection and improvement proposal of any hidden problems in the existing facility [GMP equipment diagnosis]
We investigate problematic points in the present facility in light of the latest GMP regulation, scrutinize the criticality and emergency level of the problems found, and propose any proper solution or improvement allowing the requests from the customer with the approach in the both hardware and software aspects.
- Measures and improvement proposal to the indications or observations given every inspection
We review and ascertain indications given, analyze and clear up the root causes, and offer the most recent solution and far-seeing improvements. Any items expected to be indicated in future are also clarified. Moreover, simulated inspections assuming various audits are also performed.
- Basic design / basic plan operation
We support new projects of plant constructions and FS (Feasibility Study) of the existing facilities renovation schemes, and help basic design and basic plan, layout development for budgeting, and approximate estimate calculation as a member of customers.
- Support for validation documentation
We could help customers draw up consistent and related documents based on the validation documentation system. URS, VMP, DQ, IQ, and OQ, quality risk assessment (QRA), and how to proceed with the computerized system validation could also be supported. In addition, the other support is practically made not only at the initial installation, but also in a way that allows the customer to manage the smooth periodical validation.
Value of the project
- Minimization of the investment cost for sound and lucrative business performance on CAPEX (Capital expenditure) We could clear “where” in the existing facility of the customer and “how rapid and necessary improvement” must be initiated in a way that allows the facility based on the latest GMP regulations such as PIC/S GMP. This means that minimization of the investment of CAPEX can be realized by the measures for the hardware such as equipment and instruments combined with the software such as restructuring of SOP.
- Control of excessive or complicated measures
Some proper and effective instructions can be given to the findings made by the inspection of PMDA, prefectural pharmaceutical agency, an entrusted auditor, and FDA. We could then suggest realistic measures based on the rationalistic interpretation of relative regulations and past successful examples at the site organized against the excessive method
- Practicable Concept
Practical assessment is made from every aspect of the construction, building facility, and production equipment within the limited space, construction period, and total cost in consideration of the monitoring of manufacturing environment such as cleanliness, temperature and relative humidity, differential pressure between rooms, all of which are applicable to product attributes, manufacturing facility / utility, the flow line of persons and materials, work environment, related laws (GMP, Fire Services Act).
- Reinforcement of the validation system
You could understand the relation between the respective validation documents and re-recognize the items to be described in each document. You could also conduct Quality Risk Assessment (QRA) and impact assessment, planning and programming of a high level of design review, deployment to each equipment / instruments, operation of reporting.
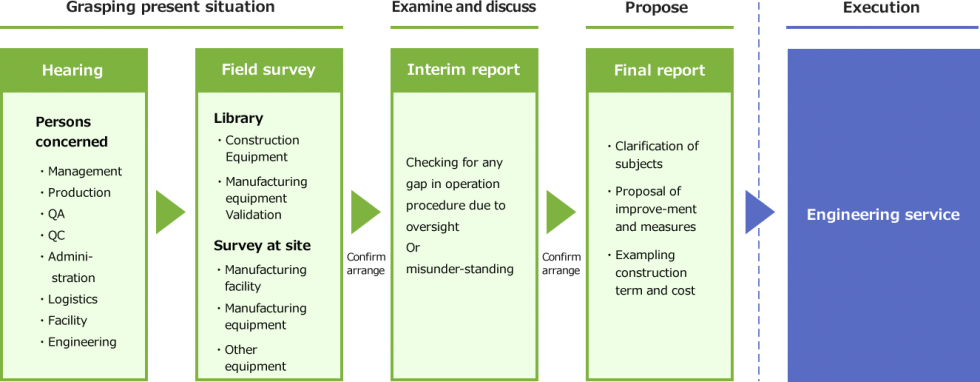
Main Workflow
Engineering Service
User’s engineering allows the thought of customers to be realized.
HES assists as a member of customers every customer to construct the pharmaceutical manufacturing and/or research facilities which are best aimed at an ideal match of the demands and real challenges of customers.
The construction in an ideal form of new plants and renovating existing facilities can be supported from the basic plan to operation under the consistent concept, and the other supporting services are also performed every stage in planning, designing, executing, and trial operation for the aimed facility.
Provided Engineering Service
- Project management
The engineers with considerable and ripe experiences in various construction/ renovation projects could scrutinize requirements demanded by the customers, consider not only the manufacturing and operating environment based on the products attributes but also the maintenance, GMP-related regulations and guidelines, and accomplish the engineering service for the whole project with logical consistency from the basic planning stage to the completion.
- Engineering management
Engineers who engaged in the manufacturing site of a pharmaceutical company for many years will support the proper selection of machinery to suit the product attributes and manufacturing characteristics, the handling method between processes, the coordination of connection with utility, the installation, and the comprehensive system construction in consideration of maintenance to periodical validation.
- Construction management
The construction manager with experience of many construction and renovation projects keeps technical neutrality between the customer and supplier, and makes various management operations for the studies of basic design and detailed design as a member of customers at each stage of design, purchase order, execution, process control, quality control, and cost control.
Value of the project
- Construction of “serviceable plant”
The construction of the pharmaceutical manufacturing facilities require not only topical knowledge and experience but also the additional capability and performance in organizing the project to be fulfilled by such various factors as construction, building facilities, manufacturing equipment for the construction. We support a person in charge of the customer’s project as a member of the team, and attempt the establishment of the serviceable plant to satisfy the customer expectation following the latest trend of the pharmaceutical industry.
- Avoidance of “omission”, “duplication” and “rework”
“Omission”, “duplication” and “rework” could be avoided in promoting the introduction plan for the manufacturing facility, when those failures result from indefinite range of installation of manufacturing equipment, inaccurate connection of such utility as electricity, air conditioning system, pharmaceutical water piping arrangements, and the lack of the consistency in handling between processes, though the specifications of each equipment are determined.
- Objective judgment and evaluation
Engineers with many experiences on-site and the latest GMP-related information provide the supporting function of the project accomplishment as a member of customers. As they can also foresee any problems that often become actualized in the practical operation at the customer site even after handed over, they point out the problems at each stage of design, purchase order, and execution. The evaluation of additional expenses can be made in proper rationalistic calculation system.
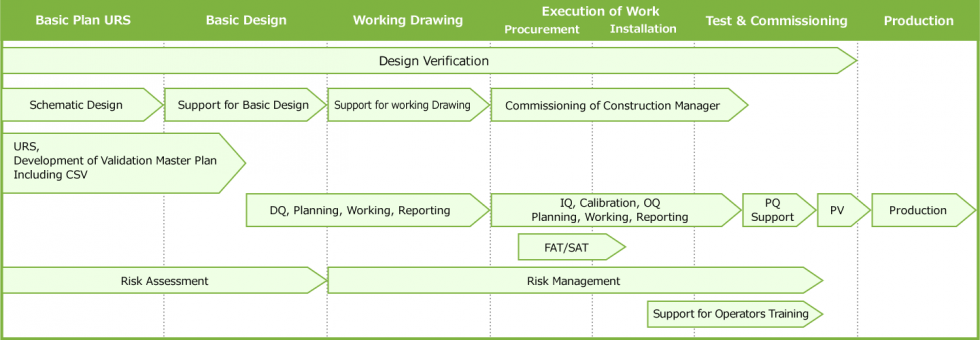
Main Workflow
contact
Contact us via email below with any questions you have.